
Applying to KAUST - Your Complete Guide for Masters & Ph.D. Programs (Upcoming Admissions)
Admissions Overview & Key Requirements
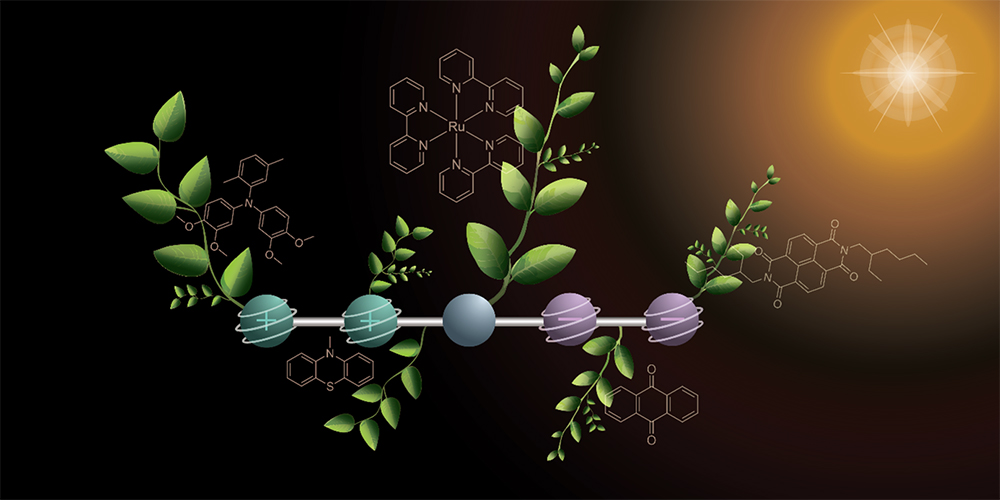
Scientists at the University of Basel have developed a specially designed molecule that could play a pivotal role in artificial photosynthesis – a process aimed at turning sunlight into sustainable fuels. The innovation, published in Nature Chemistry, allows the molecule to store two positive and two negative charges under light, mimicking the charge separation that occurs in plants.
Photosynthesis in nature uses sunlight to convert carbon dioxide into energy-rich sugars, fueling life on Earth. Researchers are striving to replicate this process to produce solar fuels such as hydrogen, methanol, and synthetic petrol – energy sources that would be carbon-neutral when burned.
The molecule, designed by a team of chemists, consists of five parts: a central unit that absorbs sunlight and two pairs of components on each side that act as electron donors and acceptors. When sunlight hits the molecule, it triggers the transfer of electrons from one side to the other, generating positive and negative charges internally.
This stored energy can then be used to split water into hydrogen and oxygen, producing clean hydrogen fuel. Unlike fossil fuels, which take millions of years to form from ancient plants and animals under intense pressure and heat, this artificial process captures solar energy instantly and emits no carbon dioxide when the hydrogen is used.
To build up the necessary four charges, researchers used two light flashes in sequence. Each flash generates a positive and a negative charge, which migrate to opposite ends of the molecule. “This stepwise excitation enables the use of significantly dimmer light, approaching the intensity of sunlight,” explains doctoral researcher Mathis Brändlin. The stored charges remain stable long enough to drive chemical reactions, such as splitting water into hydrogen and oxygen. Previously, strong laser light was required for similar experiments, which was a far cry from the vision of artificial photosynthesis.
While this breakthrough doesn’t yet constitute a fully functional artificial photosynthesis system, project lead Professor Oliver Wenger sees it as an essential piece of the puzzle. “We have implemented a key mechanism to better understand electron transfers central to artificial photosynthesis,” he says. “Our goal is to contribute to new pathways for a sustainable energy future.”
Source: University of Basel
Share

Applying to KAUST - Your Complete Guide for Masters & Ph.D. Programs (Upcoming Admissions)
Admissions Overview & Key Requirements

Erasmus Mundus Joint Master's 2026 (Upcoming Admissions)
Erasmus Mundus programs are scholarships available to students worldwide, offering fully-funded Master’s degrees to study in Europe!

Registration Opens for SAF 2025: International STEAM Azerbaijan Festival Welcomes Global Youth
The International STEAM Azerbaijan Festival (SAF) has officially opened registration for its 2025 edition!

KAIST International Graduate Admissions Spring 2026 in Korea (Fully Funded)
Applications are open for KAIST International Admissions for Master’s, Master’s-PhD Integrated, Ph.D., and Finance MBA

Young Leaders Union Conference 2025 in Paris (Fully Funded)
Join Global Changemakers in Paris! Fully Funded International Conference for Students, Professionals, and Social Leaders from All Nationalities and Fields

An mRNA cancer vaccine may offer long-term protection
A small clinical trial suggests the treatment could help keep pancreatic cancer from returning