
Registration Opens for SAF 2025: International STEAM Azerbaijan Festival Welcomes Global Youth
The International STEAM Azerbaijan Festival (SAF) has officially opened registration for its 2025 edition!
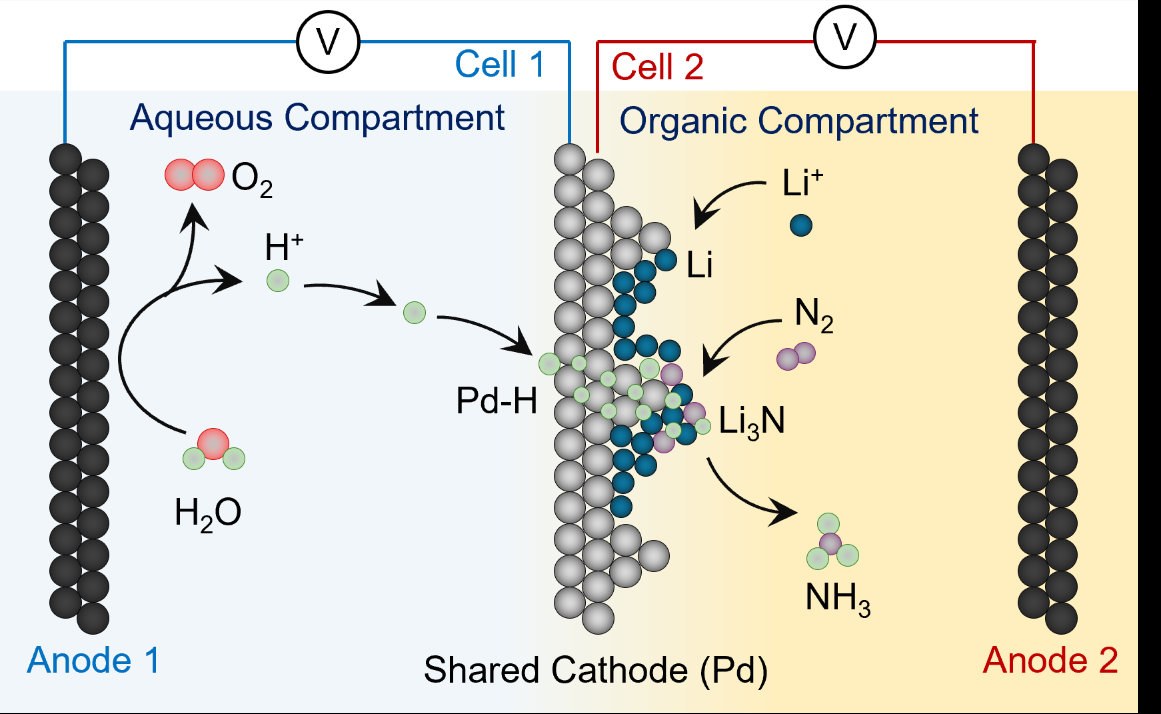
In a major step toward sustainable agriculture, researchers from the University of Bonn have developed a new, climate-friendly method for producing ammonia using only air, water, and renewable electricity. The findings, led by Prof. Dr. Nikolay Kornienko, were recently published in Nature Communications.
For over a century, the world has relied on the Haber-Bosch process to convert nitrogen from the air into ammonia, which feeds global food production. However, this method is energy-intensive and heavily dependent on fossil fuels, especially methane, releasing significant amounts of CO₂ into the atmosphere.
“The Haber-Bosch process accounts for nearly 2% of global greenhouse gas emissions,” says Prof. Kornienko. “To achieve a climate-neutral future, we must reimagine how we produce ammonia.”
The new method replaces fossil-derived hydrogen with hydrogen from water, extracted using electricity generated by solar and wind power. But making ammonia from just nitrogen and hydrogen at room temperature is chemically difficult. To overcome this, the team used a method called lithium-mediated nitrogen reduction (LiNRR). In this process, electricity is used to turn lithium ions into lithium metal. This metal then reacts with nitrogen gas from the air to form an intermediate compound. When hydrogen is added, ammonia (NH₃) is produced, and the lithium is recycled for the next reaction.
“We generally view this system as a model for the time being, as there are several practical difficulties,” says Kornienko. Because high voltage is required to reduce lithium ions to metallic lithium, energy efficiency is limited to around 25 percent. In addition. another major problem in previous green ammonia methods is the need for a hydrogen source. Although water is the ideal source of hydrogen, lithium metal, which helps the reaction, reacts violently with water. This forced scientists to use alcohols or other solvents as hydrogen donors, which get consumed during the reaction. This made the process inefficient and impractical, as these chemicals had to be sacrificed to produce ammonia.
To solve these challenges, the researchers introduced a palladium membrane that separates the lithium reaction environment from water. Palladium allows only hydrogen atoms to pass through, delivering hydrogen safely to the lithium without exposing it to water. This innovation allows hydrogen to come directly from water splitting using renewable electricity, making the process cleaner and more sustainable.
The researchers used infrared spectroscopy and mass spectrometry to verify that this really works as intended. They used a heavy isotope of hydrogen (deuterium = D) as a water source and produced ND3 instead of NH3. Conversely, the researchers labeled all molecules in the LiNRR compartment with D instead of H – as desired, NH3 was produced in this case and not ND3 as before.
Researchers Hossein Bemana and Nikolay Kornienko have already submitted a patent application for this innovative method. However, while their lab experiments successfully produced ammonia (NH₃) using only electricity, large-scale, cost-effective production from renewable energy is still far off. According to the team, ammonia output would need to be increased by a factor of 1,000 to make the process commercially viable.“We are still in the early stages,” the chemists note. “In general, research needs to be done on the reaction rates and selectivity of the system—the control of electrons to the desired target.”
Share

Registration Opens for SAF 2025: International STEAM Azerbaijan Festival Welcomes Global Youth
The International STEAM Azerbaijan Festival (SAF) has officially opened registration for its 2025 edition!

Join the Edu-live Internship Program!
Are you passionate about journalism, education, science, as well as global study and development opportunities? Do you want to be part of a dynamic media platform that brings educational and scientific news and stories to life from around the world?

Young Leaders Union Conference 2025 in Paris (Fully Funded)
Join Global Changemakers in Paris! Fully Funded International Conference for Students, Professionals, and Social Leaders from All Nationalities and Fields

An mRNA cancer vaccine may offer long-term protection
A small clinical trial suggests the treatment could help keep pancreatic cancer from returning

Yer yürəsinin daxili nüvəsində struktur dəyişiklikləri aşkar edilib
bu nəzəriyyənin doğru olmadığı məlum olub. Seismik dalğalar vasitəsilə aparılan tədqiqatda daxili nüvənin səthindəki dəyişikliklərə dair qeyri-adi məlumatlar əldə edilib.

Applying to KAUST - Your Complete Guide for Masters & Ph.D. Programs (Upcoming Admissions)
Admissions Overview & Key Requirements