
Applying to KAUST - Your Complete Guide for Masters & Ph.D. Programs (Upcoming Admissions)
Admissions Overview & Key Requirements
Researchers at the Institute for Bioengineering of Catalonia (IBEC) have created the world's simplest artificial cell capable of "chemical navigation." This groundbreaking work, published in Science Advances, demonstrates how microscopic bubbles can be chemically engineered to autonomously migrate towards specific chemical substances, much like living cells do.
The core of this discovery lies in recreating chemotaxis, a vital biological process that allows living organisms to move in response to chemical signals. From bacteria seeking food to white blood cells migrating to infection sites, chemotaxis is a fundamental survival strategy.
"What we find particularly fascinating is that this type of directed movement can occur even without the complex machinery typically involved, such as flagella or intricate signaling pathways. By recreating it in a minimal synthetic system, we aim to uncover the core principles that make such movement possible" explains Bárbara Borges Fernandes, a PhD student at IBEC and the study’s first author.
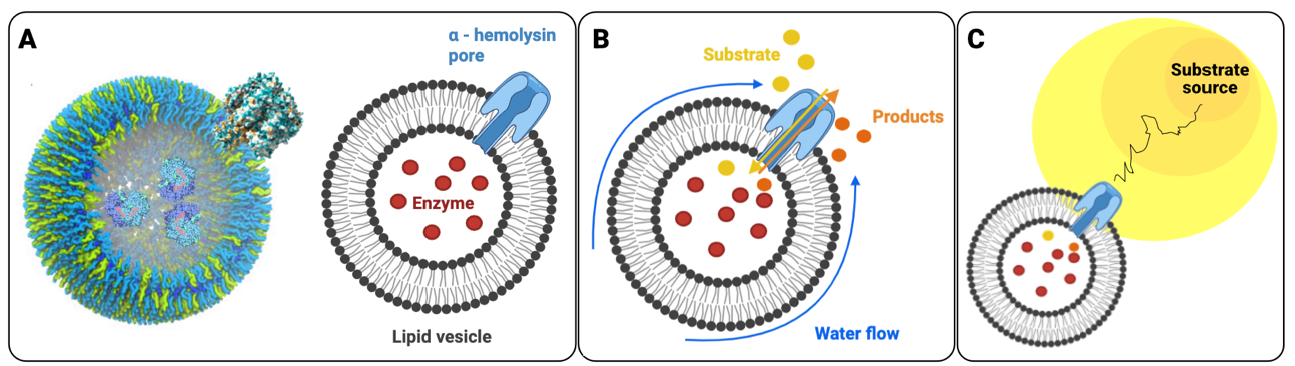
To achieve this chemical navigation, the researchers designed a "minimal cell" using lipid-based vesicles, commonly known as liposomes. These are essentially tiny, spherical bubbles made of a fatty membrane, similar to a cell's outer layer.
Here's how this "minimal cell" functions:
The research team conducted extensive experiments, tracking the movement of over 10,000 vesicles within microfluidic channels containing precise gradients of glucose or urea. They compared vesicles with varying numbers of pores to control vesicles that lacked pores entirely. Vesicles without pores drifted randomly, exhibiting only passive motion. As the number of pores increased, a chemotactic effect emerged, eventually reversing the direction of movement and actively guiding the vesicles toward regions with higher concentrations of the substrate.
Borges explains the key finding: "We observe that the control vesicles move towards lower substrate concentrations due to passive effects other than chemotaxis. As the number of pores in the vesicles increases, so does the chemotactic component. Eventually, this reverses the direction of movement, causing the vesicles to move towards areas with higher substrate concentrations." This critical reversal in direction confirms that the engineered vesicles are actively navigating towards a specific chemical “trail.”
The ability to engineer such a simple, yet purposeful, artificial cell opens up new avenues for understanding life itself. "Watch a vesicle move. Really watch it. That tiny bubble holds secrets: how cells whisper to each other, how they ship life’s cargo. But biology’s machinery is noisy, too many parts! So, we cheat," remarks Battaglia. "We rebuild the whole dance with just three things: a fatty shell, one enzyme, and a pore. No fuss. Now the hidden rules jump out. That’s the power of synthetic biology: strip a puzzle down to its bones, and suddenly you see the music in the mess. What once seemed tangled? Pure, elegant chemistry, doing more with less."
Beyond fundamental biological insights, this breakthrough provides a crucial blueprint for engineering targeted drug delivery systems. Imagine tiny "smart capsules" that can autonomously navigate through the bloodstream, following chemical signals released by diseased tissues (like cancer tumors or inflammatory sites). These capsules could then deliver medication directly to the affected area, minimizing damage to healthy cells and significantly reducing side effects. While still in the research phase, this technology holds immense potential to revolutionize how we approach medical treatments.
The study was a collaborative effort, involving contributions from José Miguel Rubí’s team at the University of Barcelona, the Institute for Physics of Living Systems and the Department of Chemistry at University College London, the University of Liverpool, the Biofisika Institute, and the Ikerbasque Foundation for Science.
Share

Applying to KAUST - Your Complete Guide for Masters & Ph.D. Programs (Upcoming Admissions)
Admissions Overview & Key Requirements

Registration Opens for SAF 2025: International STEAM Azerbaijan Festival Welcomes Global Youth
The International STEAM Azerbaijan Festival (SAF) has officially opened registration for its 2025 edition!

KAIST International Graduate Admissions Spring 2026 in Korea (Fully Funded)
Applications are open for KAIST International Admissions for Master’s, Master’s-PhD Integrated, Ph.D., and Finance MBA

Erasmus Mundus Joint Master's 2026 (Upcoming Admissions)
Erasmus Mundus programs are scholarships available to students worldwide, offering fully-funded Master’s degrees to study in Europe!

South Korea Leads OECD in Higher Education Completion for 17th Consecutive Year
With 56.2% of adults holding a college degree or higher, South Korea continues to lead the OECD in higher education attainment.

Young Leaders Union Conference 2025 in Paris (Fully Funded)
Join Global Changemakers in Paris! Fully Funded International Conference for Students, Professionals, and Social Leaders from All Nationalities and Fields